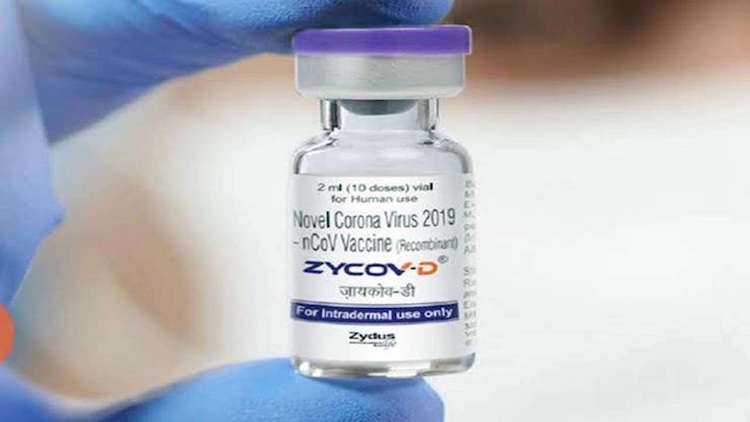
The Drugs Controller General of India (DCGI) on Friday approved Zydus Cadila’s DNA vaccine for emergency use in adults and children aged 12 years and above.
Dr Renu Swarup, Secretary, Department of Biotechnology, Government of India said, “So far the vaccines which were in the market were those which wee for above 18 years. This is the DNA vaccine that has just received emergency use authorisation is for 12 to 18 years and above. For the younger children, maybe 5 to 12 years, and even below there are different stages of the research which is still going on, and mostly all the vaccine manufacturers on different platforms are doing their trials.”
She added, “For the DNA vaccine which has just received emergency use authorisation, we’ve just been informed that this was going to take about four weeks before they can bring out the doses for the immunisation program, which children will get immunised. It will be taken by the entire NTAGI Working Group on COVID because they will have to decide, considering how much volume is going to be available. Knowing this, that this is a new technology, a new platform, it does take time for the scale-up of this vaccine.”
Latest Videos
